HEPATITIS A VIRUS (HAV)
OVERVIEW
Hepatitis A: inflammation of the liver, causing mild to severe illness. It is a highly contagious, short-term liver infection, caused by the hepatitis A virus (HAV). When the liver is inflamed or damaged, its function can be affected. Heavy alcohol use, toxins, some medications, and certain medical conditions can cause hepatitis, but it is often caused by HAV. Hepatitis A is vaccine-preventable. 
HAV is a member of the Picornaviridae family . HAV is primarily transmitted from person-to-person by the fecal-oral route either through person to person contact or consumption of contaminated food or water. The disease is closely associated with unsafe water or food, inadequate sanitation, poor personal hygiene and oral-anal sex. Although hepatitis A is not ordinarily a sexually transmitted disease, the infection rate is high among men who have sex with men due to oral-anal contact. HAV is found in the stool and blood of people who are infected. HAV is spread when someone unknowingly ingests the virus - even in microscopic amounts - through close personal contact with an infected person or through eating contaminated food or drink.
Almost everyone recovers fully from hepatitis A, with a lifelong immunity. However, a very small proportion of people infected with HAV could die from fulminant hepatitis. Safe and effective vaccines are available to prevent hepatitis A. Unlike hepatitis B and C, hepatitis A does not cause chronic liver disease, but it can cause debilitating symptoms and rarely fulminant hepatitis (acute liver failure), which is often fatal.
Hepatitis A occurs sporadically and in epidemics worldwide, with a tendency for cyclic recurrences. Epidemics related to contaminated food or water can erupt explosively. They can also be prolonged, affecting communities for months through person-to-person transmission.
GEOGRAPHICAL DISTRIBUTION
Geographical distribution areas can be characterized as having high, intermediate or low levels of HAV infection. However, infection does not always mean disease, because infected young children do not experience any noticeable symptoms. Infection is common in low- and middle-income countries with poor sanitary conditions and hygienic practices, and most children (90%) have been infected with the HAV before the age of 10 years, most often without symptoms. Infection rates are low in high-income countries with good sanitary and hygienic conditions.
Disease may occur among adolescents and adults in high-risk groups, such as people who inject drugs, men who have sex with men, people travelling to areas of high endemicity and in isolated populations. In developed countries, large outbreaks have been reported among people experiencing homelessness. In middle-income countries and regions where sanitary conditions are variable, children often escape infection in early childhood and reach adulthood without immunity.
TRANSMISSION/EXPOSURE
HAV is primarily transmitted by the faecal-oral route; that is when an uninfected person ingests food or water that has been contaminated with the faeces of an infected person. In families, this may happen through dirty hands when an infected person prepares food for family members.
The fecal-oral route of transmission can happen through:
- close person-to-person contact with an infected person
- sexual contact with an infected person
- ingestion of contaminated food or water
Waterborne outbreaks, though infrequent, are usually associated with sewage-contaminated or inadequately treated water. The virus can also be transmitted through close physical contact (such as oral-anal sex) with an infectious person.
Although viremia occurs early in infection, bloodborne transmission of HAV is known to be uncommon.
Can a person spread HAV without having symptoms?
Yes. Many people, especially children, have no symptoms but can still spread the infection. A person can transmit HAV to others up to 2 weeks before symptoms appear.
How is hepatitis A spread?
The HAV, found in the stool and blood of infected people, is spread when someone ingests the virus.
- Person-to-person contact
Hepatitis A can be spread from close, personal contact with an infected person. Hepatitis A is very contagious, and people can even spread the virus before they feel sick.
- Eating contaminated food or drink
Contamination of food with the HAV can happen at any point: growing, harvesting, processing, handling, and even after cooking. Although uncommon, foodborne outbreaks have occurred in the developed countries from people eating contaminated fresh and frozen imported food products.
What should I do if I think I have been exposed to hepatitis A virus?
If you think you have been exposed to the hepatitis A virus, call your health professional or your local or state health department as soon as possible, ideally within 2 weeks. A health professional can decide next steps based on your age and overall health.
Can I prevent infection after an exposure to the hepatitis A virus?
A single shot of the hepatitis A vaccine can help prevent hepatitis A if given within 2 weeks of exposure. Depending upon your age and health, your doctor may recommend immune globulin in addition to the hepatitis A vaccine.
If I have had hepatitis A in the past, can I get it again?
No. Once you recover from hepatitis A, you develop antibodies, protecting you for life.
How long does hepatitis A virus survive outside the body?
The hepatitis A virus can survive outside the body for months. Heating food and liquids to high temperatures can kill the virus.
SYMPTOMS
The incubation period of hepatitis A is usually 14-28 days. The symptoms can last up to 2 months and can be varied: 10-15% of symptomatic people have prolonged or relapsing disease for up to 6 months.
Symptoms of hepatitis A range from mild to severe and can include fever, malaise and/or fatigue, loss of appetite, diarrhea, nausea, abdominal discomfort (e.g.: stomach pain), dark-colored urine and jaundice (a yellowing of the eyes and skin). Not everyone who is infected will have all the symptoms.
Adults have signs and symptoms of illness more often than children. The severity of disease and fatal outcomes are higher in older age groups. Infected children under 6 years of age do not usually experience noticeable symptoms, and only 10% develop jaundice. Hepatitis A sometimes relapses, meaning the person who just recovered falls sick again with another acute episode. This is normally followed by recovery.
Most people with hepatitis A do not have long-lasting illness. The best way to prevent hepatitis A is to get vaccinated. Among older children and adults, infection is typically symptomatic. Symptoms usually occur abruptly and can include the following: fever, fatigue, loss of appetite, nausea, vomiting, abdominal pain, dark urine, diarrhea, clay/light-colored stool, joint pain, jaundice. Most (70%) of infections in children younger than the age of 6 are not accompanied by symptoms. When symptoms are present, young children typically do not have jaundice; most (>70%) older children and adults with HAV infection have this symptom.
People who get hepatitis A may feel sick for a few weeks to several months but usually recover completely and do not have lasting liver damage. In rare cases, hepatitis A can cause liver failure and even death; this is more common in older people and in people with other serious health issues, such as chronic liver disease.
Not everyone with hepatitis A has symptoms. Adults are more likely to have symptoms than children. If symptoms develop, they usually appear 2 to 7 weeks after infection. Symptoms usually last less than 2 months, although some people can be ill for as long as 6 months.
WHO IS AT RISK?
Anyone who has not been vaccinated or previously infected can get infected with HAV. In areas where the virus is widespread (high endemicity), most hepatitis A infections occur during early childhood. Although anyone can get hepatitis A, certain groups of people are at higher risk for getting infected and for having severe disease if they do get hepatitis A.
Risk factors include:
- poor sanitation;
- living in a household with an infected person;
- being a sexual partner of someone with acute hepatitis A infection;
- use of recreational drugs;
- sex between men;
- travelling to areas of high endemicity without being immunized;
- people with occupational risk for exposure;
- people with chronic liver disease, including hepatitis B and C;
- people with HIV.
The last 2 are people at increased risk for severe disease from hepatitis A infection.
INCUBATION PERIOD FOR HAV: 28 days (range: 15-50 days).
DIAGNOSIS
How is hepatitis A diagnosed?
A doctor can determine if you have hepatitis A by discussing your symptoms and ordering a blood test that can tell whether you have been recently infected with the virus that causes hepatitis A.
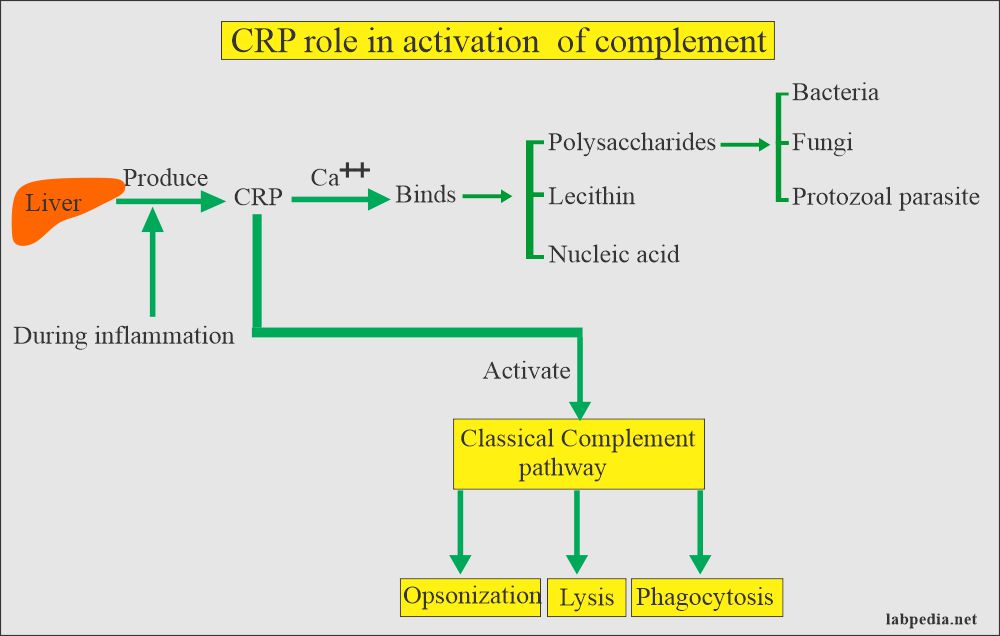
Cases of hepatitis A are not clinically distinguishable from other types of acute viral hepatitis. Specific diagnosis is made by the detection of HAV-specific immunoglobulin IgM antibodies in the blood. Additional tests include reverse transcriptase polymerase chain reaction (RT-PCR) to detect the hepatitis A virus RNA and may require specialized laboratory facilities.
The case definition for acute hepatitis A:
- clinical criteria: an acute illness with a discrete onset of any sign or symptom consistent with acute viral hepatitis (e.g., fever, headache, malaise, anorexia, nausea, vomiting, diarrhea, abdominal pain, or dark urine)
AND
a) jaundice or elevated total bilirubin levels ≥ 3.0 mg/dL, OR
b) elevated serum alanine aminotransferase (ALT) levels >200 IU/L
AND
c) the absence of a more likely diagnosis
LABORATORY CRITERIA FOR DIAGNOSIS
Confirmatory laboratory evidence:
- Immunoglobulin M (IgM) antibody to hepatitis A virus (anti-HAV) positive,
OR
- Nucleic acid amplification test (NAAT; such as polymerase chain reaction [PCR] or genotyping) for hepatitis A virus RNA positive.
CASE CLASSIFICATION - CONFIRMED
- A case that meets the clinical criteria and is IgM anti-HAV positive+ OR
- A case that has hepatitis A virus RNA detected by NAAT (such as PCR or genotyping ) OR
- A case that meets the clinical criteria and occurs in a person who had contact (e.g., household or sexual) with a laboratory-confirmed hepatitis A case 15-50 days prior to onset of symptoms
CAN HEPATITIS A BECOME CHRONIC?
No. Hepatitis A does not become a chronic, long-term infection.
CAN SOMEONE BECOME RE-INFECTED WITH THE HEPATITIS A VIRUS?
No. Immunoglobulin G antibodies (IgG) to the hepatitis A virus, which appear early in the course of infection, provide lifelong protection against the disease.
Hepatitis A virus detection:
- highlighting acute-phase anti-HAV IgM antibodies by ELISA method (transaminases go up as well); acute-phase antibodies appear from the debut and last for about 10 weeks;
- after 10 weeks: anti-HAV IgG that lasts until the end of life, their presence reflecting either disease or vaccine.
HAV IgG/IgM Rapid Test example: The onsite HAV IgG/IgM Rapid Test is usually a lateral flow chromatographic immunoassay for the qualitative detection and differentiation of antibodies (IgG and IgM) to Hepatitis A virus in human serum, plasma or whole blood. It is intended to be used as a screening test and provides a preliminary test result to aid in the diagnosis of HAV infection.
The presence of anti-HAV IgM in blood samples suggests an acute or recent HAV infection. In most infected individuals, anti-HAV IgM rapidly increases in titer over a period of 4-6 weeks post infection and then declines to non-detectable levels within 3 to 6 months. Anti-HAV IgG can be detected at the onset of symptoms and remain elevated throughout an individual's life. Protective immunity from an infection with HAV is indicated by an anti-HAV IgG level ≥ 20-33 mIU/mL, though the presence of anti-HAV IgG ≥ 20-33 mIU/mL does not necessarily ensure protection from a future HAV infection. A patient without protective levels of anti-HAV IgG (<20-33 mIU/mL) is considered a risk of acquiring an HAV infection.
The OnSite HAV IgG/IgM Rapid Test can usually be performed within 15 minutes by minimally skilled personnel without the use of laboratory equipment. Test principle: the test strip in the cassette device consists of: 1) a burgundy coloured conjugate pad containing HAV antigens conjugated with colloidal gold (HAV conjugates) and a control antibody conjugated with colloidal gold; 2) a nitrocellulose membrane strip containing two test lines (G and M lines) and a control line (C line). The G line is pre-coated with mouse anti-human IgG for detection of anti-HAV IgG. The M line is pre-coated with mouse anti-human IgM for detection of anti-HAV IgM. The C line is pre-coated with a control antibody.
When an adequate volume of test specimen and sample diluent is dispensed into the sample well and buffer well, respectively, the specimen migrates by capillary action across the test strip. If anti-HAV IgG is present in the specimen, it will bind to the HAV conjugates. The immunocomplex is then captured on the membrane by the pre-coated mouse anti-human IgG forming a burgundy coloured G-line, indicating an HAV IgG positive test result. If anti-HAV IgM is present in the specimen it will bind to the HAV conjugates. The immunocomplex is then captured on the membrane by the pre-coated mouse anti-human IgM forming a burgundy coloured M line, indicating an HAV IgM positive test result.
Absence of any test lines (G or M) suggests a negative result. The test contains an internal control (C line) which should exhibit a burgundy coloured line of the immunocomplex of the control antibodies, regardless of colour development of the test lines (G and M). If no control line (C line) develops, the test result is invalid and the specimen must be retested with another device.
TREATMENT
There is no specific treatment for hepatitis A. Recovery from symptoms following infection may be slow and can take several weeks or months. Hospitalization is unnecessary in the absence of acute liver failure. Therapy is aimed at maintaining comfort and adequate nutritional balance, including replacement of fluids that are lost from vomiting and diarrhea.
HOW IS HAV INFECTION PREVENTED?
Vaccination with the full, two-dose series of hepatitis A vaccine is the best way to prevent infection. Hepatitis A vaccines are available for use in people 1 year of age and older.
Immune globulin can provide protection against hepatitis A, both pre- and postexposure.
PREVENTION
Improved sanitation, food safety and immunization are the most effective ways to combat hepatitis A.
The spread of hepatitis A can be reduced by:
- adequate supplies of safe drinking water;
- proper disposal of sewage within communities; and
- personal hygiene practices such as regular handwashing before meals and after going to the bathroom.
HEPATITIS A VACCINATION
WHO SHOULD BE VACCINATED AGAINST HEPATITIS A?
Children
- All children aged 12-23 months
- Unvaccinated children and adolescents aged 2-18 years old
People at increased risk for HAV infection
- International travelers
- Men who have sex with men
- People who use injection or noninjection drugs
- People with occupational risk for exposure
- People who anticipate close personal contact with an infected person
- People experiencing lack of hygienic conditions
People at increased risk for severe disease from HAV infection
- People with chronic liver disease
- People with human immunodeficiency virus infection
Other people recommended for vaccination
- Pregnant women at risk for HAV infection or severe outcome from HAV infection
- Any person who requests vaccination
Vaccination during outbreaks
- Unvaccinated people in outbreak settings who are at risk for HAV infection or at risk for severe disease from HAV
What if an infant receives the first dose of hepatitis A vaccine at an age younger than 12 months?
Although no known harm is associated with giving hepatitis A vaccine to infants, the hepatitis A vaccine dose(s) administered prior to 12 months of age might result in a suboptimal immune response, particularly in infants with passively acquired maternal antibody. Therefore, hepatitis A vaccine dose(s) administered at <12 months of age are not considered valid doses.
The two-dose hepatitis A vaccine series should be initiated when the child is at least 1 year of age.
How long does protection from hepatitis A vaccine last?
The exact duration of protection against hepatitis A virus infection after vaccination is unknown.
Anti-HAV has been shown to persist for at least 20 years in most people receiving the 2-dose series as infants <2 years of age, those vaccinated with a 3-dose series as young children (aged 3-6 years), and adults receiving the entire vaccine series during adulthood.
Can hepatitis A vaccine be administered concurrently with other vaccines?
Yes. Hepatitis B, diphtheria, poliovirus (oral and inactivated), tetanus, typhoid (oral and intramuscular), cholera, rabies, and yellow fever vaccines can be given at the same time that hepatitis A vaccine is given.
In studies among young children, simultaneous administration of hepatitis A vaccine did not affect the immunogenicity or reactogenicity of diphtheria-tetanus-acellular pertussis; inactivated polio; measles, mumps, rubella (MMR); hepatitis B; and Haemophilus influenzae type b vaccines.
Can a patient receive the first dose of hepatitis A vaccine from one manufacturer and the second (last) dose from another manufacturer? Ideally, doses of vaccine in a series come from the same manufacturer; however, if this is not possible or if the manufacturer of doses given previously is unknown, providers should administer the vaccine that they have available. The dose should be considered valid and does not need to be repeated.
What should be done if the second (last) dose of hepatitis A vaccine is delayed?
The second dose should be given as soon as possible. Even if the second dose is delayed, the first dose does not need to be repeated.
IMMUNE GLOBULIN
What is immune globulin?
Immune globulin is a substance made from human blood plasma that contains antibodies, which are the body's natural defense against infection. Injections of immune globulin may be given under certain circumstances, like when someone is too young to get vaccinated or can't get vaccinated because of a previous, life-threatening reaction to the hepatitis A vaccine or vaccine component. Unlike the hepatitis A vaccine, immune globulin does not provide long-term protection against infection.
POSTEXPOSURE PROPHYLAXIS FOR HEPATITIS A
What should be done when a case of hepatitis A is found in a setting providing services to children or adults (e.g., a school, hospital, office setting, corrections facility, or homeless shelter)?
- Postexposure prophylaxis (PEP) is not routinely indicated when a single case occurs in a school or work setting and the source of infection is outside of the setting.
- When a person who has HAV infection is admitted to a hospital, staff members should not routinely be administered PEP; instead, appropriate infection control practices should be emphasized.
- PEP should be administered to people who have close contact with index patients if an epidemiologic investigation indicates HAV transmission has occurred among students in a school or among patients or between patients and staff members in a hospital.
- PEP should be considered for all previously unvaccinated residents and employees when a confirmed hepatitis A case occurs in a setting where close personal contact occurs regularly and hygiene standards are difficult to maintain (e.g., correctional facility, homeless shelter, various facilities). In a setting containing multiple enclosed units or sections (e.g., prison ward), PEP administration should be limited only to people in the area where there is exposure risk.
Do immunocompromised people require additional protection after being exposed to someone with hepatitis A?
Yes. People who are immunocompromised or have chronic liver disease and who have been exposed to hepatitis A virus within the past 2 weeks and have not previously completed the 2-dose hepatitis A vaccination series should receive both immune globulin and hepatitis A vaccine simultaneously in a different anatomic site (e.g., separate limbs) as soon as possible after exposure. When the dose of hepatitis A vaccine administered is the first dose the exposed individual has received, a second dose should be administered 6 months after the first for long-term protection.
Should pregannt women at increased risk for exposure to the hepatitis A virus (HAV) receive postexposure prophylaxis?
Women with increased likelihood of exposure to HAV during pregnancy can be administered immune globulin. There has been no observed increase in maternal or infant adverse events after hepatitis A vaccination or IG administration in pregnancy.
HEPATITIS A AND INTERNATIONAL TRAVEL
Who should get the hepatitis A vaccine before travelling internationally?
All unvaccinated people, along with those who have never had hepatitis A, should be vaccinated before traveling to countries where hepatitis A is common. Travelers to countries where hepatitis A is common are still at risk. International travelers have been infected, even though they regularly washed their hands and were careful about what they drank and ate. Those who are too young or can't get vaccinated because of a previous, life-threatening reaction to the hepatitis A vaccine or vaccine component should receive immune globulin.
How soon before travel should I get the hepatitis A vaccine?
You should get the first dose of hepatitis A vaccine as soon as you plan international travel to a country where hepatitis A is common. The vaccine will provide some protection even if you get vaccinated closer to departure. For older adults (age >40 years), people who are immunocompromised, and people with chronic liver disease or other chronic medical conditions the health-care provider may consider, based on several factors, giving an injection of immune globulin at the same time in different limbs.
What should I do if I am traveling internationally but cannot receive hepatitis A vaccine?
People who are allergic to a vaccine component or are younger than 6 months should receive a single dose of immune globulin before traveling to a country where hepatitis A is common. Immune globulin provides effective protection against hepatitis A virus infection for up to 2 months, depending on the dosage given. If you are staying longer than 2 months, you can get another dose of immune globulin during your visit for continued protection against hepatitis A.




















.jpg)









