Serology - Immunology
Agglutination Kits & Serology Reagents: there are many options of latex serology kits on the market. Common serological tests to detect in serum:
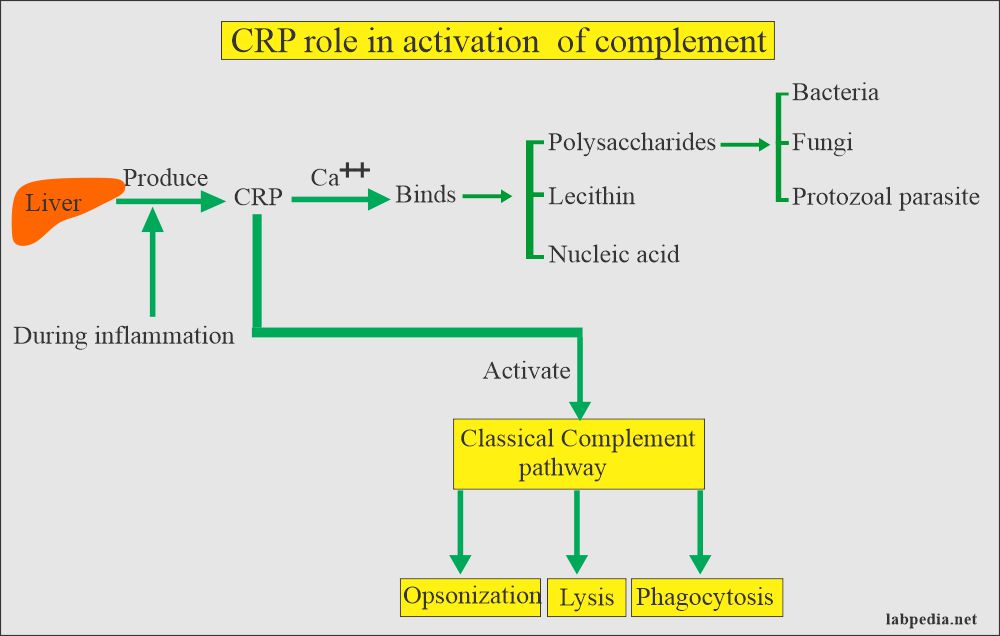
- ASLO/ASO: Anti-streptolysin-O; - CRP: C-Reactive Protein;
- RF: Rheumatoid Factor.
The latex assays are available in different kit sizes, with positive and negative controls provided. The full kits include all the required consumables. They use the common agglutination method for efficient, reliable testing, to ascertain the causes of inflammation, rheumatoid disorders and streptococcal infections. Latex agglutination testing is sometimes referred to as latex serology.
Agglutination occurs when an antigen comes into contact with its corresponding antibody. The rapid test kits are used to identify streptococcal infections, levels of C-reactive protein that would indicate high levels of inflammation in the body and rheumatoid factors that would indicate a diagnosis of rheumatoid arthritis.
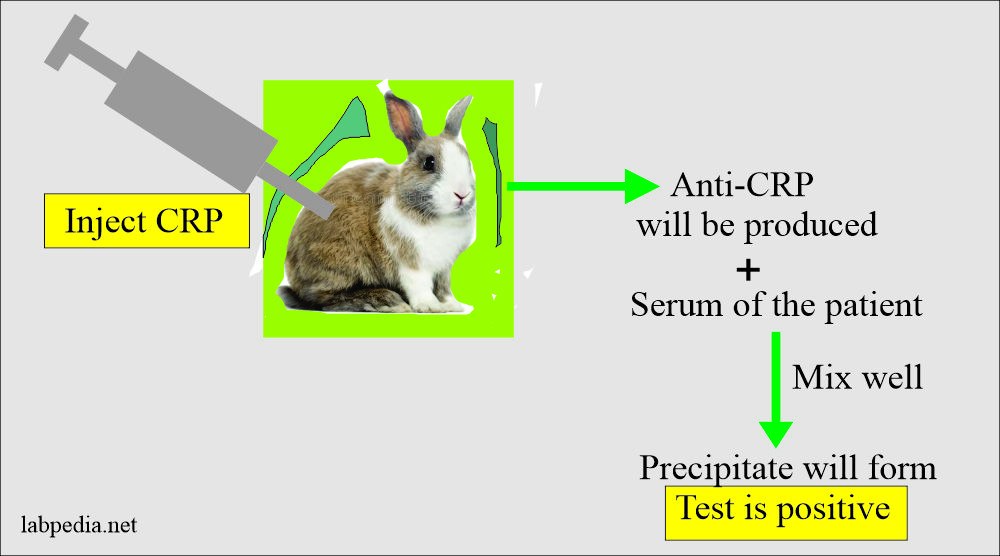
Latex serology tests are used to ascertain whether specific antigens or antibodies are present in a sample. This is done by applying the samples to the latex beads. If the suspected substance is present in the sample, the latex beads clump together (agglutinate). The results of latex serology tests can be obtained within 15 minutes to an hour, allowing a rapid diagnosis to be made, which is obviously a significant advantage.
These slide agglutination assay tests are used for the qualitative and semi-quantitative determination of ASLO, CRP and RF in human serum. The simple, five-step procedure provides easy-to-read results in 2 minutes. No initial dilution of patient samples is required. Kits usually include ASLO/CRP/RF latex reagents, reactive control, nonreactive control, 100 disposable stirrer pipettes and disposable cards. The latex reagent does not require additional preparation. MATERIAL REQUIRED: automatic pipettes, mechanical rotator, adjustable at 100 r.p.m, laboratory alarm clock.
QUALITY CONTROL: Positive and negative controls should be run daily following the steps outlined in the Qualitative Test, in order to check the optimal reactivity of the reagent. The positive control should produce clear agglutination. If the expected result is not obtained, you must not use it.
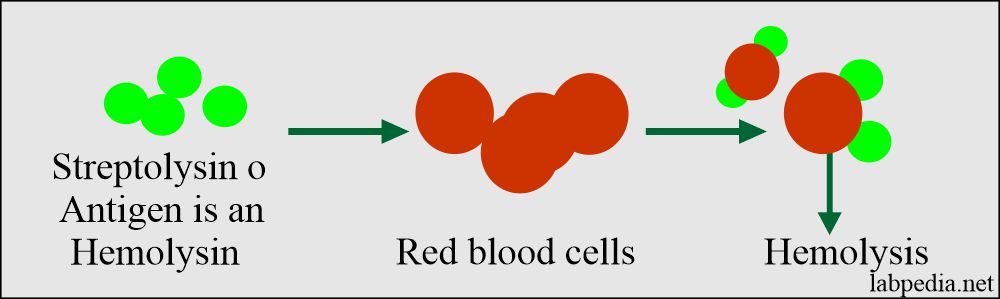
ASO/ASLO: slide agglutination test for the qualitative and semi-quantitative detection of streptolysin antibodies in human serum. Analytical sensitivity: 200 (+/-50) IU/mL.
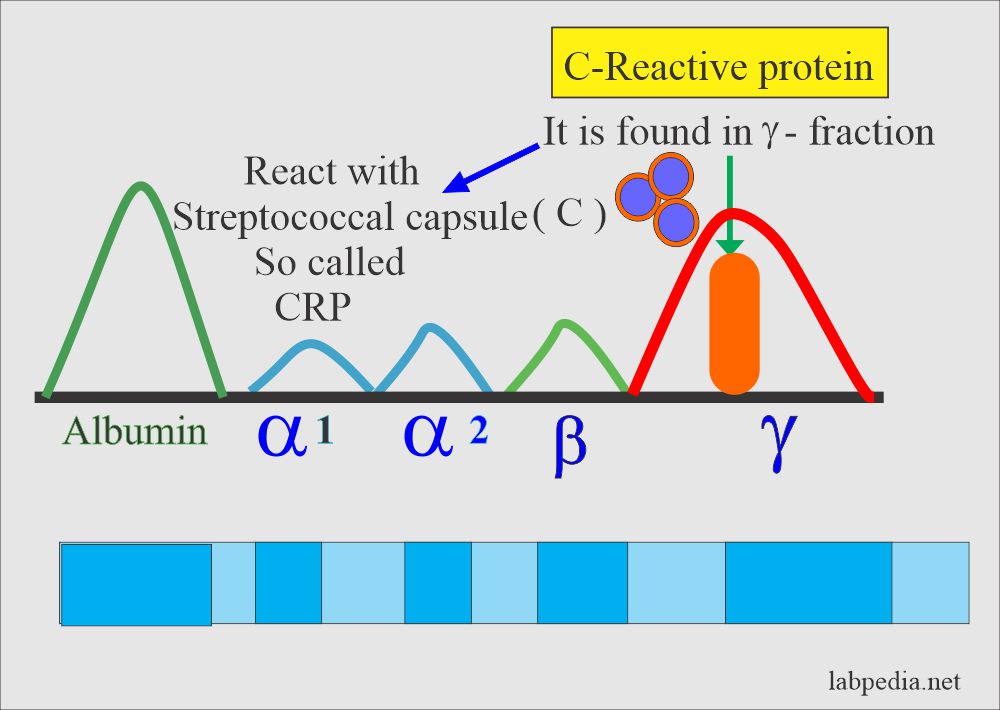
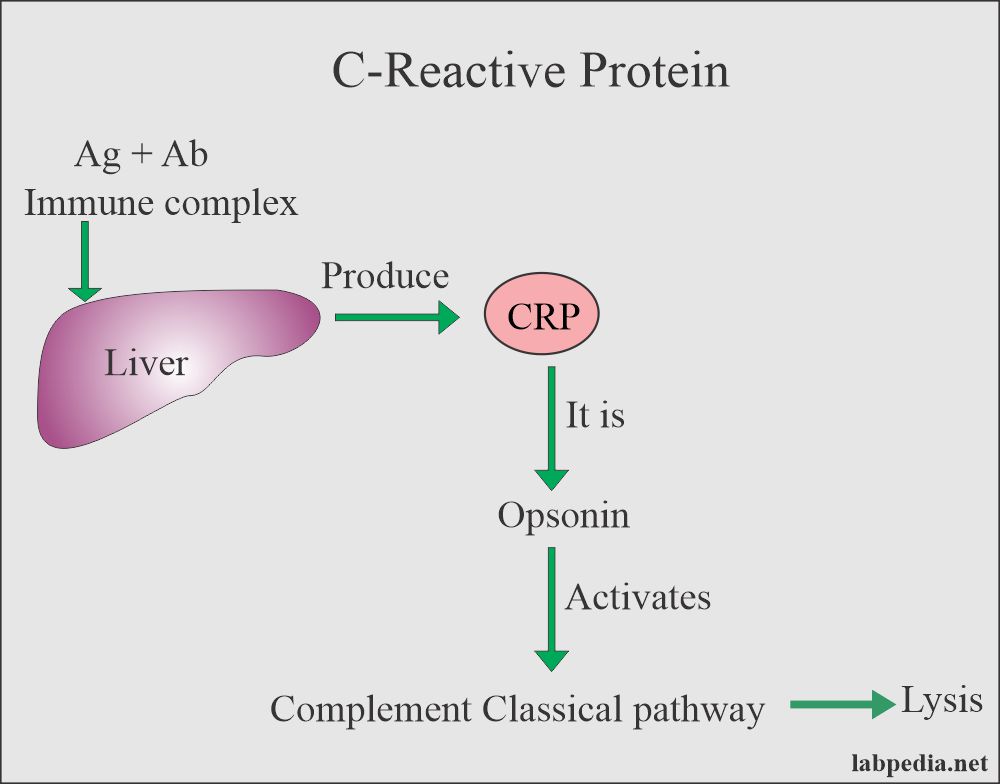
CRP: slide agglutination test for the qualitative and semi-quantitative detection of C-reactive protein in human serum. Analytical sensitivity: 6 mg/L.
RF: slide agglutination test for the qualitative and semi-quantitative detection of rheumatoid factors in human serum. Analytical sensitivity: 8 IU/mL.
SOURCES OF ERROR: Bacterial contamination of controls and specimens as well as freezing and thawing of the ASLO/CRP/RF-latex reagents may lead to false positive results. Traces of detergent in the test cards may give false positive results. Preferably the used cards would not be reused. If cards are reused, wash them first under tap water until all reactants are removed and then with distilled water. Allow to air dry, avoiding the use of organic solvents as they may impair the special finish on the slide.
The ASLO/CRP/RF-latex antigen must not be used beyond its expiry date because a prolonged storage can affect the sensitivity of the suspension. Do not allow reagents in the kit to get in contact with skin or mucous membranes.
Reagents are stable if stored at 2-8ºC up to expiration date. Allow the suspension to reach to room temperature and mix gently prior to use. Do not freeze. Frozen reagents could change the functionality of the test. Reagent and Controls are ready for use and stable until the expiry date stated on the label. Do not use haemolysed, lipaemic or contaminated serum for testing. Hemoglobin (<10 g/L), bilirubin (<20 mg/dL) and lipemia (<10 g/L) do not interfere. Other substances (rare) may interfere. SAMPLES: must be fresh, clear serum. After the clear serum has been separated it may be stored at 2-8ºC for up to one week or longer periods at -20ºC, before testing. Undiluted samples should be used. Do not use plasma since fibrinogen can form non-specific agglutination. The sensitivity of the test may be reduced at low temperatures. The best results are achieved at 15-25ºC.
PROCEDURAL STEPS for the QUALITATIVE TEST:
1. Bring the test reagents and samples to room temperature.
2. Transfer 50µl/1 drop of the patient's serum into one of the circles of the test card. Dispense 1 drop of positive control and 1 drop of negative control into two additional circles.
3. Gently shake the suspension of the latex reagent (ASLO/CRP/RF), then using the pipette, aspirate dropper several times to obtain a thorough mixing. Add 1 drop/50µl reagent of the suspension to the same test circle with the patient's serum and to those with the controls.
4. Using the stirrers, mix the serum and the latex reagent and spread them. Mix the contents of each circle while spreading over the entire area enclosed by the ring. Use separate stirrers for each mixture.
5. Rotate the test card/slide for 2 minutes by means of a mechanical rotator (100r.p.m.) and observe immediately under a suitable light source for any degree of agglutination.
READING THE LATEX SLIDES:
- Nonreactive: smooth suspension with no visible agglutination, as shown by the negative control.
- Reactive: any degree of agglutination visible macroscopically (best to check under a suitable light).
The delays in reading the results may generate in overestimation of the antibody present.
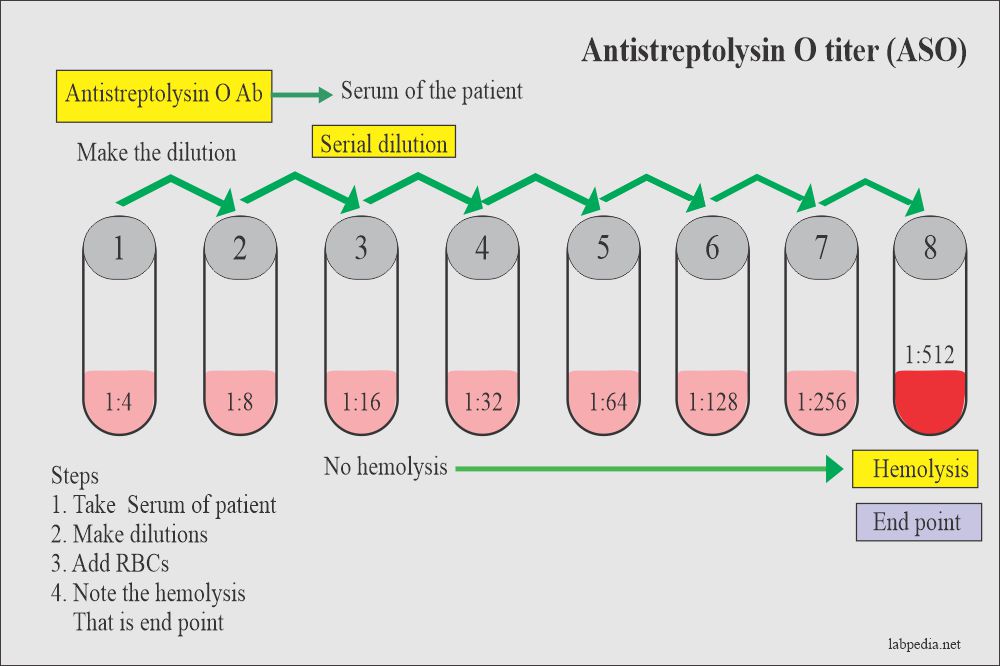
For the SEMI-QUANTITATIVE TEST:
- Dilute the sample to be tested following the 2-fold dilutions, as follows: 1/2, 1/4, 1/8, 1/16, 1/32. Follow the other steps as in the quantitative test.
How to make the dilutions: it is up to the biologist, for as long as it is correctly diluted mathematically.
Example of dilution 1/2: 50µL non-diluted sample + 50µL dilution liquid (physiological serum/distilled water), then transfer 1 drop/50µL from the ASLO/CRP/RF latex reagent over the mixture. The other steps are as in the quantitative test.
Reading is the same for both the Qualitative and for the Semi-Quantitative Test:
- Negative/Non-reactive: no agglutination;
- Positive/Reactive: visible agglutination.
The titer of the specimen for the Semi-Quantitative test is reported as the highest dilution that shows reactivity. The next higher dilution should be negative. If the highest dilution tested is reactive, repeat the test using the next dilution.
The results obtained for the Semi-Quantitative Test do not replace the fully-automated quantitative tests available on the market. The levels obtained following the Semi-Quantitative Test procedure are considered only "approximate", not exact values, hence the naming of "Semi-Quantitative Test".
The approximate ASLO/CRP/RF levels (IU/mL) present in the sample may be obtained by multiplying the titer of the last positive dilution by the minimum detectable unit (analytical sensitivity) corresponding to each reagent type.
Reading the Semi-Quantitative tests:
ASLO:
- 1/2 dilution shows reactivity => ASLO level: 2 x 200 = 400 IU/mL
- 1/4 dilution shows reactivity => ASLO level: 4 x 200 = 800 IU/mL
- 1/8 dilution shows reactivity => ASLO level: 8 x 200 = 1600 IU/mL
- 1/16 dilution shows reactivity => ASLO level: 16 x 200 = 3200 IU/mL
1/4 reactive and 1/8 non-reactive => ASLO level: 800-1600; ASLO>800 and <1600.
CRP:
- 1/2 dilution shows reactivity => CRP level: 2 x 6 = 12 mg/L
- 1/4 dilution shows reactivity => CRP level: 4 x 6 = 24 mg/L
- 1/8 dilution shows reactivity => CRP level: 8 x 6 = 48 mg/L
- 1/16 dilution shows reactivity => CRP level: 16 x 6 = 96 mg/L
1/2 reactive and 1/4 non-reactive => CRP level: 12-24; CRP>12 and <24.
RF:
- 1/2 dilution shows reactivity => RF level: 2 x 8 = 16 IU/mL
- 1/4 dilution shows reactivity => RF level: 4 x 8 = 32 IU/mL
- 1/8 dilution shows reactivity => RF level: 8 x 8 = 64 IU/mL
- 1/16 dilution shows reactivity => RF level: 16 x 8 = 128 IU/mL.
1/8 reactive and 1/16 non-reactive => RF level: 64-128; RF>64 and <128.